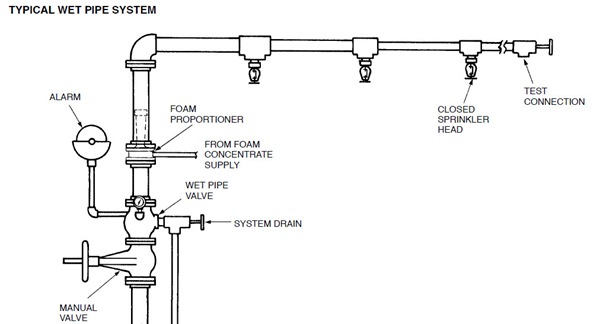
This system uses automatic closed-head sprinklers that are attached to a piping system containing water. Water discharges immediately from those sprinklers opened by a fire. A flow of water through the valve sounds an alarm.
Wet pipe systems should not be used where freezing conditions are likely to damage piping. In systems using AFFF concentrate, the piping to the sprinkler heads can be pre-primed with foam solution to enable immediate effective foam discharge. AFFF solution in contact with steel pipe may gradually lose its fire effectiveness. Samples of this solution should be checked on an annual basis and replenished as needed. A test discharge connection is recommended downstream from the proportioner and should be located to fill a maximum portion of the sprinkler system piping. The test connection should be of sufficient size to meet the minimum flow rate for the particular proportioner. This type of system is the most reliable, simplest, and fastest responding of all closed-head sprinkler systems. Conversions of water systems to foam can usually be accomplished easily. (Local codes and regulations should be investigated prior to conversion.)
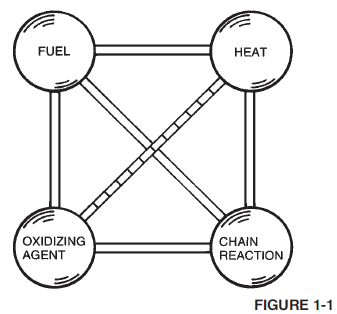
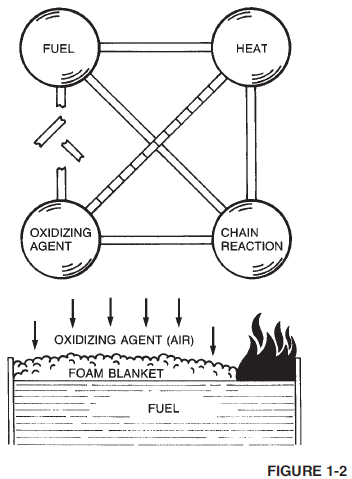
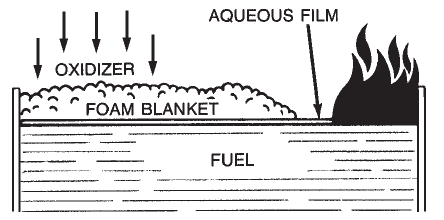 Aqueous Film-Forming Foam (AFFF) is a completely synthetic foam. It consists of combinations of fluorochemical and hydrocarbon surfactants combined with high boiling point solvents and water. Surfactants are chemicals that have the ability to alter the surface properties of water. Fluorochemical surfactants alter these properties in such a way that a thin film (Figure 1-7) can spread on a hydrocarbon fuel (such as gasoline) even though the aqueous film is more dense than the fuel.
Aqueous Film-Forming Foam (AFFF) is a completely synthetic foam. It consists of combinations of fluorochemical and hydrocarbon surfactants combined with high boiling point solvents and water. Surfactants are chemicals that have the ability to alter the surface properties of water. Fluorochemical surfactants alter these properties in such a way that a thin film (Figure 1-7) can spread on a hydrocarbon fuel (such as gasoline) even though the aqueous film is more dense than the fuel. 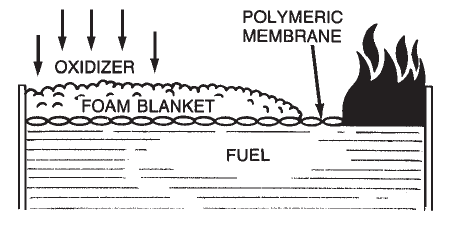 Alcohol-Resistant Concentrate (ARC) produces a foam that is effective on fuels such as methyl alcohol, ethyl alcohol, and acetone which have appreciable water solubility or miscibility. Standard foam agents are mixtures of chemicals (natural or synthetic) whose bubbles collapse when applied to water soluble fuels. These fuels are said to be foam destructive. The early alcohol-resistant foams were based on mixtures of protein foams and chemicals called metal soaps. These chemicals are hydrophobic or water repellent. The most current alcohol-resistant concentrates are based on AFFF concentrates to which a water soluble polymer (polysaccharide) has been added. When these foam agents are applied to a water soluble fuel such as methyl alcohol, a polymeric membrane (Figure 1-8) is formed between the foam and the water soluble fuel. When this foam agent is used on a conventional (water insoluble) hydrocarbon fuel, it functions as an AFFF foam by forming an aqueous film at the fuel/air interface. Since the polymer is a naturally occurring chemical, small amounts of an antimicrobial agent are added to prevent biological degradation.
Alcohol-Resistant Concentrate (ARC) produces a foam that is effective on fuels such as methyl alcohol, ethyl alcohol, and acetone which have appreciable water solubility or miscibility. Standard foam agents are mixtures of chemicals (natural or synthetic) whose bubbles collapse when applied to water soluble fuels. These fuels are said to be foam destructive. The early alcohol-resistant foams were based on mixtures of protein foams and chemicals called metal soaps. These chemicals are hydrophobic or water repellent. The most current alcohol-resistant concentrates are based on AFFF concentrates to which a water soluble polymer (polysaccharide) has been added. When these foam agents are applied to a water soluble fuel such as methyl alcohol, a polymeric membrane (Figure 1-8) is formed between the foam and the water soluble fuel. When this foam agent is used on a conventional (water insoluble) hydrocarbon fuel, it functions as an AFFF foam by forming an aqueous film at the fuel/air interface. Since the polymer is a naturally occurring chemical, small amounts of an antimicrobial agent are added to prevent biological degradation.