In order to understand how foam suppresses fire, it is first necessary to understand the process of combustion.
Combustion is a process where fuel undergoes a rapid exothermic chemical reaction (release of heat) with an oxidizing agent, usually air, resulting in the formation of products of combustion and energy (fire).
A fuel is any material that can be oxidized; it can be a solid, liquid, or gas and is generally organic in nature, i.e., composed mostly of carbon, hydrogen, or oxygen. The products of combustion of an organic fuel (assuming complete combustion) are carbon dioxide and water. The energy released may be in the form of heat or light, or the combination of heat and light (fire).
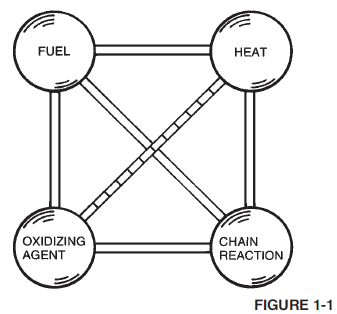
The chemical reaction is not a simple one-step reaction, but is a chain reaction resulting in a number of interdependent chemical reactions. Figure 1-1 depicts the four requirements for combustion using the “fire tetrahedron.”
It follows that any method for extinguishing fire must involve one or more of the following techniques:
- Remove heat at a faster rate than it is released.
- Separate the fuel from the oxidizing agent.
- Dilute the vapour-phase concentration of the fuel and/or oxidizing agent below that necessary for combustion.
- Terminate the chemical chain-reaction sequence.
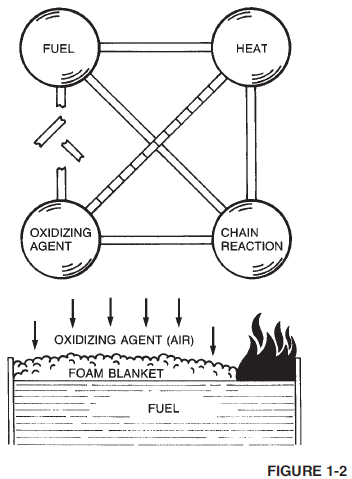
Fire fighting foam is an aggregate of gas-filled bubbles (Figure 1-2) formed from aqueous solutions of specially formulated, liquid agent concentrates. The gas used is usually air, but certain applications use an inert gas.
Since foam is lighter than flammable and combustible liquids, it floats on the fuel surface producing a continuous blanket that suppresses fire by separating flammable vapours and oxygen as shown in Figure 1-2. Because foam is a water-bearing material, it also cools the fuel surface.
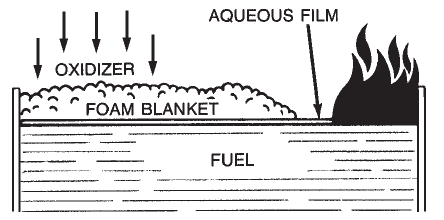 Aqueous Film-Forming Foam (AFFF) is a completely synthetic foam. It consists of combinations of fluorochemical and hydrocarbon surfactants combined with high boiling point solvents and water. Surfactants are chemicals that have the ability to alter the surface properties of water. Fluorochemical surfactants alter these properties in such a way that a thin film (Figure 1-7) can spread on a hydrocarbon fuel (such as gasoline) even though the aqueous film is more dense than the fuel.
Aqueous Film-Forming Foam (AFFF) is a completely synthetic foam. It consists of combinations of fluorochemical and hydrocarbon surfactants combined with high boiling point solvents and water. Surfactants are chemicals that have the ability to alter the surface properties of water. Fluorochemical surfactants alter these properties in such a way that a thin film (Figure 1-7) can spread on a hydrocarbon fuel (such as gasoline) even though the aqueous film is more dense than the fuel. 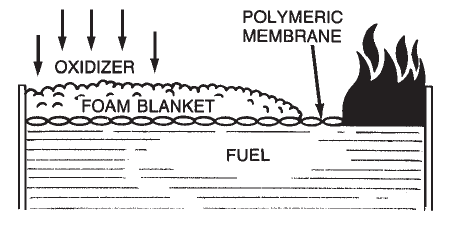 Alcohol-Resistant Concentrate (ARC) produces a foam that is effective on fuels such as methyl alcohol, ethyl alcohol, and acetone which have appreciable water solubility or miscibility. Standard foam agents are mixtures of chemicals (natural or synthetic) whose bubbles collapse when applied to water soluble fuels. These fuels are said to be foam destructive. The early alcohol-resistant foams were based on mixtures of protein foams and chemicals called metal soaps. These chemicals are hydrophobic or water repellent. The most current alcohol-resistant concentrates are based on AFFF concentrates to which a water soluble polymer (polysaccharide) has been added. When these foam agents are applied to a water soluble fuel such as methyl alcohol, a polymeric membrane (Figure 1-8) is formed between the foam and the water soluble fuel. When this foam agent is used on a conventional (water insoluble) hydrocarbon fuel, it functions as an AFFF foam by forming an aqueous film at the fuel/air interface. Since the polymer is a naturally occurring chemical, small amounts of an antimicrobial agent are added to prevent biological degradation.
Alcohol-Resistant Concentrate (ARC) produces a foam that is effective on fuels such as methyl alcohol, ethyl alcohol, and acetone which have appreciable water solubility or miscibility. Standard foam agents are mixtures of chemicals (natural or synthetic) whose bubbles collapse when applied to water soluble fuels. These fuels are said to be foam destructive. The early alcohol-resistant foams were based on mixtures of protein foams and chemicals called metal soaps. These chemicals are hydrophobic or water repellent. The most current alcohol-resistant concentrates are based on AFFF concentrates to which a water soluble polymer (polysaccharide) has been added. When these foam agents are applied to a water soluble fuel such as methyl alcohol, a polymeric membrane (Figure 1-8) is formed between the foam and the water soluble fuel. When this foam agent is used on a conventional (water insoluble) hydrocarbon fuel, it functions as an AFFF foam by forming an aqueous film at the fuel/air interface. Since the polymer is a naturally occurring chemical, small amounts of an antimicrobial agent are added to prevent biological degradation. 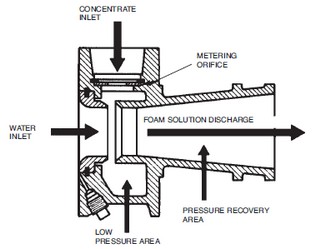 Balanced Pressure Proportioning is the most common method used for fixed system applications where pressure or flow can vary with demand. There are two basic types – Bladder Tanks and Pump Systems using Balanced Pressure Proportioners. All balanced pressure systems use a modified venturi device called a proportioner or ratio controller. The proportioners are available in a variety of sizes and styles to match required flow ranges and pipe sizes. As water flows through the proportioner nozzle, a low pressure area is created. It is in this low pressure area that the pressurized concentrate mixes with the water stream. A metering orifice, at the concentrate inlet, regulates the rate of concentrate flow and thus determines the percentage of concentrate in the foam solution. Balanced pressure proportioning systems require the foam concentrate pressure to be balanced with the water pressure at the proportioner inlets. This balance meters the proper amount of foam concentrate into the water stream.
Balanced Pressure Proportioning is the most common method used for fixed system applications where pressure or flow can vary with demand. There are two basic types – Bladder Tanks and Pump Systems using Balanced Pressure Proportioners. All balanced pressure systems use a modified venturi device called a proportioner or ratio controller. The proportioners are available in a variety of sizes and styles to match required flow ranges and pipe sizes. As water flows through the proportioner nozzle, a low pressure area is created. It is in this low pressure area that the pressurized concentrate mixes with the water stream. A metering orifice, at the concentrate inlet, regulates the rate of concentrate flow and thus determines the percentage of concentrate in the foam solution. Balanced pressure proportioning systems require the foam concentrate pressure to be balanced with the water pressure at the proportioner inlets. This balance meters the proper amount of foam concentrate into the water stream.

 The in-line balanced pressure proportioner is similar to the pump skid previously described except that it is a separate assembly that offers the advantage of proportioning the foam concentrate at a location remote from the tank and pump. Like the pump skid, the proportioner assembly incorporates an automatic pressure balancing valve, duplex gauge, and hand-operated valves for optional manual pressure regulation. A pressure control valve, located in the return line to the foam concentrate storage tank, maintains constant pressure in the supply manifold that is 15 to 20 psi (103 to 138 kPa) higher than the water pressure to the proportioner.
The in-line balanced pressure proportioner is similar to the pump skid previously described except that it is a separate assembly that offers the advantage of proportioning the foam concentrate at a location remote from the tank and pump. Like the pump skid, the proportioner assembly incorporates an automatic pressure balancing valve, duplex gauge, and hand-operated valves for optional manual pressure regulation. A pressure control valve, located in the return line to the foam concentrate storage tank, maintains constant pressure in the supply manifold that is 15 to 20 psi (103 to 138 kPa) higher than the water pressure to the proportioner. 
 Balanced Pressure Proportioning is the most common method used for fixed system applications where pressure or flow can vary with demand.There are two basic types – Bladder Tanks and Pump Systems using Balanced Pressure Proportioners.
Balanced Pressure Proportioning is the most common method used for fixed system applications where pressure or flow can vary with demand.There are two basic types – Bladder Tanks and Pump Systems using Balanced Pressure Proportioners. ANSULITE® 3×3 Low-Viscosity AR-AFFF (Alcohol-Resistant Aqueous Film-Forming Foam) Concentrate is formulated using a newly patented and proprietary technology. The foam concentrate has a dramatically reduced viscosity as compared to other listed polar-solvent type AR-AFFF concentrates on the market. This reduced viscosity enhances performance in all types of foam proportioning equipment including inline eductors, balanced pressure systems, and built-in systems aboard CFR vehicles.
ANSULITE® 3×3 Low-Viscosity AR-AFFF (Alcohol-Resistant Aqueous Film-Forming Foam) Concentrate is formulated using a newly patented and proprietary technology. The foam concentrate has a dramatically reduced viscosity as compared to other listed polar-solvent type AR-AFFF concentrates on the market. This reduced viscosity enhances performance in all types of foam proportioning equipment including inline eductors, balanced pressure systems, and built-in systems aboard CFR vehicles.